How To Determine Stability Of A Compound
Introduction
Electrons have no stock-still position in atoms, compounds and molecules (run across epitome below) but take probabilities of being found in certain spaces (orbitals). Resonance forms illustrate areas of higher probabilities (electron densities). This is like holding your hat in either your correct manus or your left. The term Resonance is practical when in that location are two or more possibilities bachelor. Resonance structures practice not modify the relative positions of the atoms like your arms in the metaphor. The skeleton of the Lewis Structure remains the same, merely the electron locations change.
"PICK THE Right ARROW FOR THE JOB"
Nigh arrows in chemistry cannot exist used interchangeably and care must be given to selecting the correct arrow for the job.
- ↔↔: A double headed arrow on both ends of the arrow between Lewis structures is used to show their inter-connectivity
- ⇌⇌: Double harpoons are used to designate equilibria
- ⇀⇀: A single harpoon on one end betoken the movement ofaneelectron
- →→: A double headed pointer on i stop is used to bespeak the movement ofiielectrons
Example 1: Ozone
Consider ozone (O3)
SOLUTION


An animation of how one tin do a resonance with ozone by moving electrons
Delocalization and Resonance Structures Rules
In resonance structures, the electrons are able to move to help stabilize the molecule. This movement of the electrons is called delocalization.
-
- Resonance structures should have the same number of electrons, do not add or subtract any electrons. (check the number of electrons by simply counting them).
- All resonance structures must follow the rules of writing Lewis Structures.
- The hybridization of the construction must stay the same.
- The skeleton of the structure tin not be inverse (only the electrons motility).
- Resonance structures must besides have the aforementioned corporeality of lone pairs.
Formal Charge
Fifty-fifty though the structures look the aforementioned, the formal charge (FC) may not be. Formal charges are charges that are assigned to a specific atom in a molecule. If computed correctly, the overall formal charge of the molecule should be the same equally the oxidation charge of the molecule (the charge when you lot write out the empirical and molecular formula). We desire to choose the resonance structure with the least formal charges that add upwardly to nada or the charge of the overall molecule. The equation for finding Formal Charge is:
Formal Charge = (number of valence electrons in gratis orbital) – (number of lone-pair electrons) – ( [latex] \frac{ane}{2} [/latex] number bond pair electrons)
The formal charge has to equal the molecule'due south overall accuse,e.g., the [latex] CNS^- [/latex] has an overall charge of -ane, so the Lewis construction'southward formal charge has to equal -1.
Example 2: Thiocyanate Ion
Consider the thiocyanate ([latex] CNS^- [/latex]) ion.
SOLUTION
1. Find the Lewis Structure of the molecule. (Retrieve the Lewis Construction rules.)

two. Resonance: All elements want an octet, and nosotros can practice that in multiple ways past moving the terminal atom's electrons around (bonds too).

3. Assign Formal Charges
Formal Charge = (number of valence electrons in complimentary orbital) – (number of lone-pair electrons) – ( [latex] \frac{1}{ii} [/latex] number bail pair electrons)
Remember to decide the number of valence electron each atom has before assigning Formal Charges
C = 4 valence e–, N = 5 valence e–, S = half dozen valence e–, also add an actress electron for the (-1) charge. The full of valence electrons is 16.

iv. Observe the almost ideal resonance structure. (Notation: It is the one with the to the lowest degree formal charges that adds upwardly to nada or to the molecule's overall charge.)

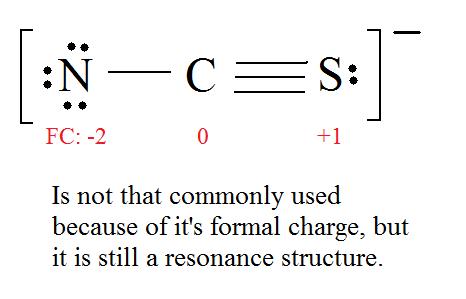
five. At present we have to expect at electronegativity for the "Right" Lewis structure.
The about electronegative cantlet usually has the negative formal accuse, while the least electronegative atom usually has the positive formal charges.

Resonance Hybrids
Resonance Structures are a representation of a Resonance Hybrid, which is the combination of all resonance structures. The resonance structure with the Formal Accuse closest to zero is the nigh accustomed structure, however, the correct Lewis construction is actually a combination of all the resonance structures and is not solely depict as one.
-
- Depict the Lewis Construction & Resonance for the molecule (using solid lines for bonds).
- Where there can be a double or triple bail, draw a dotted line (—–) for a bond.
- Draw merely the lone pairs plant in all resonance structures, do not include the lone pairs that are not on all of the resonance structures.
Note: The right Lewis structure is actually a combination of all the resonance structures and hence is not solely described as one.
Example 3: Carbonate Ion
Consider the carbonate ion: CO3 two –
SOLUTION

Pace 1: Draw the Lewis Structure & Resonance.

Step 2: Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed.

Step 3: Add only the lone pairs found onALL resonance structures.

The bottom is the finished resonance hybrid forCO3 2- .
Rules for estimating stability of resonance structures
-
- The greater the number of covalent bonds, the greater the stability since more atoms volition take complete octets
- The construction with the to the lowest degreenumber of formal charges is more stable
- The structure with the least separation of formal charge is more stable
- A structure with a negative charge on the more than electronegative atom will exist more than stable
- Positive charges on the least electronegative cantlet (about electropositive) is more stable
- Resonance forms that are equivalent have no difference in stability and contribute equally (eg. benzene)
Example 4: Benzene and Aminophenol
Benzene is an extremely stable molecule and information technology is deemed for its geometry and molecular orbital interaction, only most chiefly it's due to its resonance structures. The delocalized electrons in the benzene ring brand the molecule very stable and with its characteristics of a nucleophile, information technology will react with a potent electrophile only and afterwards the showtime reactivity, the substituted benzene will depend on its resonance to straight the next position for the reaction to add a second substituent.

Aminophenol is a very stable molecule that is present in most biological systems, mainly in proteins. By studies of NMR spectroscopy and 10-Ray crystallography it is confirmed that the stability of the amide is due to resonance which through molecular orbital interaction creates almost a double bond between the Nitrogen and the carbon.

Case 5: Multiple Resonance of other Molecules
Molecules with multiple resonance forms

Some structural resonance conformations are the major contributor or the dominant forms that the molecule exists. For case, if we look at the above rules for estimating the stability of a molecule, we see that for the third molecule the beginning and second forms are the major contributors for the overall stability of the molecule. The nitrogen is more than electronegative than carbon and so, it can handle the negative accuse more than carbon. A carbon with a negative charge is the least favorable conformation for the molecule to exist, so the last resonance form contributes very little for the stability of the Ion.
The Hybrid Resonance forms evidence the dissimilar Lewis structures with the electron been delocalized. This is very important for the reactivity of chlorobenzene considering in the presence of an electrophile it will react and the formation of some other bond will exist directed and decide by resonance. The long pair of electrons delocalized in the aromatic substituted ring is where it tin can potentially class a new bond with an electrophile, every bit it is shown in that location are three possible places that reactivity can take place, the outset to react will have place at theparaposition with respect to the chloro substituent so to eitherorthoposition.
References
-
- Petrucci, Ralph H., et al. General Chemical science: Principles and Mod Applications. New Jersey: Pearson Prentice Hall, 2007.
- Ahmad, Wan-Yaacob and Zakaria, Mat B. "Cartoon Lewis Structures from Lewis Symbols: A Direct Electron Pairing Arroyo." Journal of Chemical Education: Journal 77.3.
Exercises
-
- True or False, The picture show below is a resonance structure?

-
- Depict the Lewis Dot Structure for SO iv 2 – and all possible resonance structures. Which of the following resonance construction is not favored among the Lewis Structures? Explain why. Assign Formal Charges.
- Describe the Lewis Dot Construction for CH3COO – and all possible resonance structures. Assign Formal Charges. Choose the most favorable Lewis Structure.
- Depict the Lewis Dot Structure for H PO iii 2 – and all possible resonance structures. Assign Formal Charges.
- Draw the Lewis Dot Structure forCHO2 ane – and all possible resonance structures. Assign Formal Charges.
- Draw the Resonance Hybrid Construction for P O iv 3 – .
- Depict the Resonance Hybrid Construction for Northward O three – .
Problems #two


Answers
Problems #2
Testify Respond
Contributors
-
- Sharon Wei (UCD), Liza Chu (UCD)
Source: https://courses.lumenlearning.com/suny-mcc-organicchemistry/chapter/the-predicted-stabilities-of-resonance-contributors/

0 Response to "How To Determine Stability Of A Compound"
Post a Comment